Dian Li, Jinfeng Yang, Bingyan Liu, Jianxian Gong,* and Zhen Yang*
Org. Lett. 2021, 23(12)
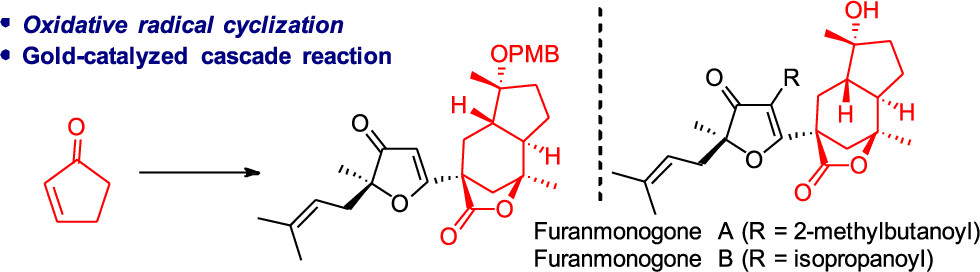
A strategy for the stereoselective synthesis of desacyl furanmonogones A and B has been achieved. The key steps in this synthesis are (1) an Fe(ClO4)3-mediated oxidative radical cyclization for construction of a cis-fused [5–6]-bicyclic core with a bridged lactone substitute, (2) a phosphorane-mediated rearrangement to convert the cis-fused [5–6]-bicyclic core to the corresponding trans-fused [5–6]-bicyclic core, and (3) a Au-catalyzed cascade reaction for formation of the 4,5-seco-3(2H)-furanone motif.