Pengpeng Zhang, Yuanhe Li, Zhiming Yan, Jianxian Gong*, Zhen Yang*
J. Org. Chem. 2019, 84(24), 15958-15971
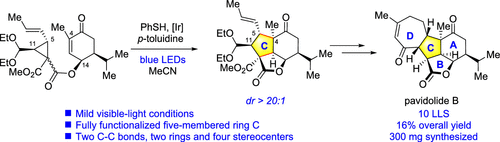
The development of an efficient strategy for the asymmetric total synthesis of the bioactive marine natural product (−)-pavidolide B is described in detail. The development process and detours leading to the key thiyl-radical-mediated [3 + 2] annulation reaction, which constructed the central C ring with four contiguous stereogenic centers in one step, are depicted. Subsequently, the seven-membered D ring is constructed via a ring-closing metathesis reaction followed by a Rh(III)-catalyzed isomerization. This strategy enables the total synthesis of (−)-pavidolide B in the longest linear sequence of 10 steps.