Xin-Ting Liang, Bao-Chuan Sun, Chang Liu, Yuan-He Li, Nan Zhang, Qian-Qian Xu, Zhong-Chao Zhang, Yi-Xin Han, Jia-Hua Chen,* and Zhen Yang*
J. Org. Chem. 2021, 86(3)
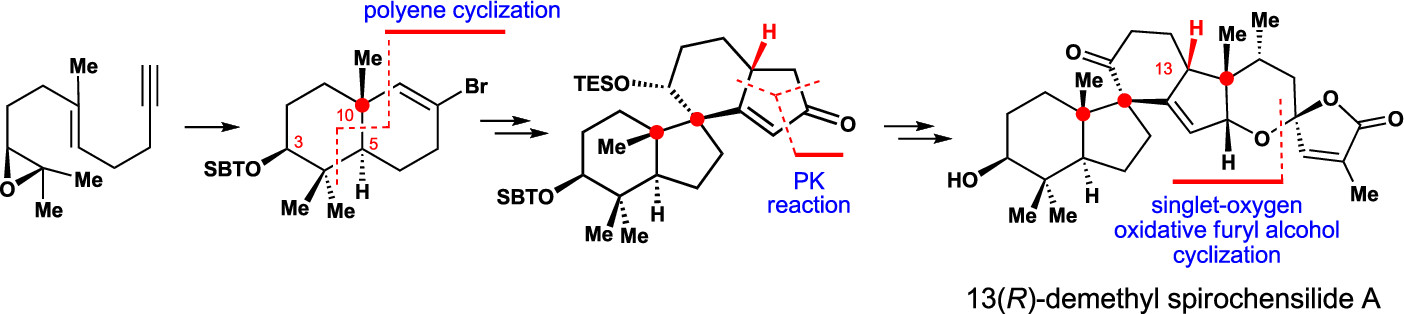
A concise and diastereoselective construction of the ABCD ring system of spirochensilide A is described. The key steps of this synthesis are a semipinacol rearrangement reaction to stereoselectively construct the AB ring system bearing two vicinal quaternary chiral centers and a Co-mediated Pauson–Khand reaction to form the spiro-based bicyclic CD ring system. This chemistry leads to the stereoselective synthesis of 13(R)-demethyl spirochensilide A, paving the way for the first asymmetric total synthesis of (−)-spirochensilide A.